Patient safety in medical research is a paramount concern that remains a cornerstone of ethical inquiry and innovation in healthcare. As medical research funding faces unprecedented challenges, particularly through disruptions like federal funding freezes, the safeguards designed to protect study participants become increasingly vital. Research ethics oversight, specifically through Institutional Review Boards (IRBs), aims to ensure that human subjects are treated with the utmost respect and safety throughout a study’s lifecycle. Not only do rigorous assessments help mitigate risks associated with participation, but they also uphold public trust in medical advancements, such as those seen in recent Harvard medical studies. Understanding the delicate balance of patient safety and research progress is essential, especially in discussions surrounding the NIH funding impact on ongoing projects.
The integrity of research involving human subjects hinges on the prioritization of participant welfare within the clinical trial framework. This realm often encompasses various aspects, such as ethical governance and the mechanisms of research oversight that protect individuals giving invaluable consent to participate. By employing rigorous review processes, including those conducted by IRBs, researchers strive to navigate the complexities of medical experimentation while ensuring a just balance between innovation and safety. The current landscape of research funding challenges poses risks that could undermine the dedication of researchers committed to safeguarding participant interests and wellbeing.
The Significance of Patient Safety in Medical Research
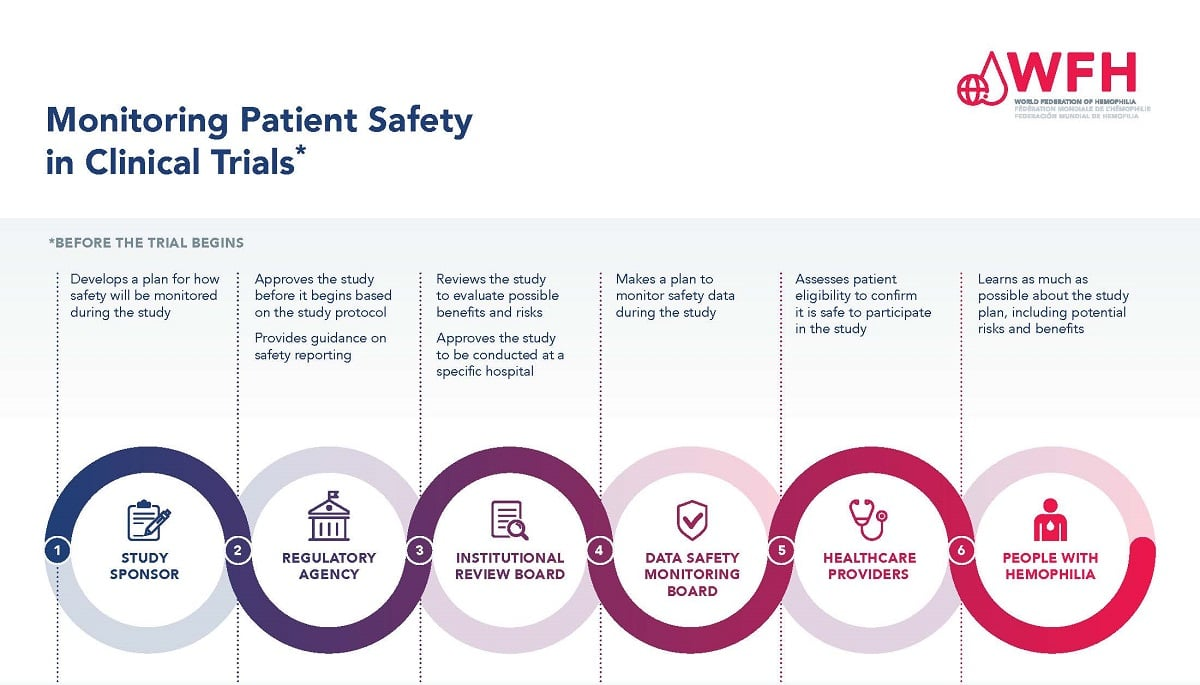
Patient safety is a cornerstone of ethical medical research, serving as the first priority of any clinical trial or study. To ensure that individuals participating in research are adequately protected, rigorous regulations and oversight mechanisms are put in place. These measures include the establishment of Institutional Review Boards (IRBs), which oversee studies involving human subjects, safeguarding their well-being. By conducting thorough assessments of research plans, IRBs ensure that potential risks are minimized, informed consent is obtained, and that participants are adequately informed of the potential benefits and burdens associated with the study. A well-functioning IRB can truly make the difference between safe, ethical research and work that perhaps falls short of these essential standards.
As highlighted in various studies, including those funded by the NIH, maintaining a strong focus on patient safety not only builds trust between researchers and participants but also enhances the overall quality of the research. A supportive funding environment contributes significantly to this safety net. When financial resources become scarce—due to cuts or freezes in funding—research institutions may struggle to uphold the rigorous standards required for ethical oversight, leading to potential gaps in safety protocols. This cascade effect underscores the profound implications that funding decisions can have on the well-being of individuals involved in medical research.
Moreover, the historical context surrounding patient safety, including lessons learned from past medical abuses, underscores its importance. Events such as the Tuskegee Syphilis Study or unethical research practices during wartime have forever changed how society views informed consent and participation in medical studies. These historical atrocities serve as a constant reminder of the need for robust patient protection measures, reverberating into modern-day policies and ethical considerations in research. The creation of strong oversight frameworks, funded adequately through governmental and private avenues, ensures that similar occurrences do not happen again.
In a rapidly evolving medical landscape, researchers must navigate complex ethical considerations while innovating new treatments and interventions. The continual oversight provided by IRBs not only emphasizes patient safety but also plays a crucial role in shaping public perceptions of medical research. Lawmakers and funding bodies must recognize the essential link between funding, ethical practices, and patient safety, ensuring that future medical advancements do not compromise on the rights and welfare of research participants.
How IRB and Ethical Oversight Protect Patients
Institutional Review Boards (IRBs) are vital components of ethical medical research, serving to protect the welfare of participants. The primary responsibility of an IRB is to conduct thorough reviews of research proposals, ensuring that the risks are justified by the potential benefits. This includes assessing the study design, recruitment strategy, and informed consent process to ensure participants’ rights are prioritized. Furthermore, IRBs play a pivotal role in the continuous monitoring of trials, ensuring that any adverse events are promptly reported and addressed, thereby protecting patient safety throughout the study’s duration.
The ethical oversight provided by IRBs is crucial, especially in collaborative studies that may involve multiple institutions. With the NIH’s mandate for the use of a single IRB for multi-site studies, the efficiency of ethical oversight can be enhanced while maintaining high safety standards. This collaborative approach minimizes redundancy in the review process, streamlining the path for innovation while still ensuring that participants’ rights and safety remain uncompromised. By addressing the ethical dimensions of research comprehensively, IRBs foster a culture of trust and accountability between researchers and participants.
In addition to their role in oversight, IRBs also play a critical part in educating researchers about ethical standards and practices. This includes facilitating training sessions that emphasize the importance of patient safety, informed consent, and the potential impact of research findings. As research becomes increasingly complex, the responsibility on researchers to adhere to ethical principles doubles. The proactive involvement of IRBs helps cultivate a strong ethical foundation across research teams, encouraging investigators to prioritize patient safety as they navigate their studies. This is especially important as funding cuts threaten to dilute these oversight functions, making continued investment in IRB processes essential.
The ability of IRBs to adapt to emerging ethical concerns in response to evolving scientific methodologies demonstrates their importance. As new medical technologies emerge, the ethical challenges associated with them, such as those seen in genetic research or AI applications in healthcare, require nuanced understanding and oversight. This adaptability ensures that as new potentials for research arise, patient safety remains paramount, reinforcing the integrity of the research process and maintaining the public’s trust.
The Impact of Research Funding on Patient Safety Initiatives
Research funding is a critical pillar in the continuation and enhancement of patient safety initiatives within medical studies. Without sufficient financial support, institutions face increased challenges in maintaining the rigorous oversight required to protect participants. The recent cuts to NIH funding illustrate the adverse effects such freezes can have, not only on ongoing research projects but also on the broader framework of patient safety protocols. As funding diminishes, the resources available for staffing IRBs, conducting necessary training, and implementing comprehensive safety measures are severely limited, which can place research participants at risk of harm.
Organizations that rely heavily on external funding, like those supported by NIH or private grants, often face uncertainties that can directly impact ongoing studies. The interruption in funding can halt projects mid-study, leaving both researchers and participants in precarious positions. For instance, when studies are unable to recruit necessary participants or expand clinical sites due to funding restrictions, the potential benefits of those studies may never materialize. Moreover, the cancellation of research initiatives can perpetuate public skepticism about the research industry and its commitment to patient safety, ultimately stymying future advancements in medical treatments.
It is also essential to consider that adequate research funding facilitates innovation in patient safety interventions. For example, institutions that have access to robust funding can invest in advanced training for IRB members, expanding their understanding of evolving research ethics and ensuring they are equipped to manage new challenges effectively. Moreover, funding helps to foster collaboration among institutions, as seen in efforts like the SMART IRB initiative. By pooling resources and sharing expertise, institutions can develop and implement best practices for patient safety that transcend local boundaries.
In conclusion, the intricate relationship between research funding and patient safety cannot be overstated. As funding sources fluctuate, the implications for ethical oversight are significant; hence, it is imperative for policymakers and stakeholders to recognize the importance of sustained financial support for medical research. Protecting patient safety is a collective responsibility that hinges on maintaining robust research funding, which in turn fortifies the overall integrity of medical research.
Historical Context of Patient Protection in Medical Research
Understanding the history of patient protection in medical research is crucial to appreciating the current ethical standards and oversight mechanisms in place. Historical breaches of ethical conduct, such as the infamous Tuskegee Syphilis Study, serve as stark reminders of the potential for harm when participant welfare is neglected. The ethical outrage that arose from such cases catalyzed the formation of stringent regulations and the establishment of IRBs tasked with safeguarding the rights and safety of participants in research studies. These historical lessons have been instrumental in shaping current patient protection frameworks, ensuring that researchers are held accountable for their practices and that participants are treated with dignity and respect.
In the modern context, landmark legislation, such as the National Research Act of 1974, was established to address these ethical concerns and create national standards for the protection of human subjects. This legislation paved the way for a system of ethics oversight that is anchored in respect for individuals’ rights and the fundamental principle of informed consent. As researchers today navigate complex ethical dilemmas, understanding the historical context allows them to recognize the ongoing importance of ethical practices and the necessity for continued vigilance in safeguarding participant rights.
Moreover, the evolution of patient protection policies reflects a broader societal commitment to address historical injustices in medical research. The development of ethical standards in research has not only been about preventing future abuses but also about rebuilding trust with the communities that have been affected by past transgressions. Gaining the confidence of diverse populations—particularly those historically subjected to unethical practices—requires ongoing engagement, transparency, and accountability from researchers and institutions alike. This commitment to ethical integrity in medical research is critical, as it impacts not only the participants in current studies but also shapes public perception and trust in the larger research enterprise.
In conclusion, acknowledging the historical context of patient protection in medical research informs our current approach to ethical oversight and patient safety. By learning from past mistakes and actively promoting ethical standards, the medical research community can foster a safe environment for participants, mitigating skepticism, and paving the way for future advancements in healthcare and medical treatments.
The Role of NIH Funding in Enhancing Medical Research Ethics
NIH funding has a long-standing reputation for supporting quality medical research that prioritizes ethical considerations and patient safety. By providing substantial financial resources, the NIH enables research institutions to hire skilled personnel, support IRB functions, and implement comprehensive training for researchers in ethical practices. This level of funding is instrumental in fostering a research environment that prioritizes the well-being of participants and adheres to the highest ethical standards. In turn, this not only protects the participants but also enhances the overall quality of research outcomes, as studies conducted with a strong ethical framework often yield more reliable and impactful results.
Furthermore, NIH-funded projects are often held to stringent ethical guidelines that promote responsible conduct in research. For example, studies that receive NIH support must comply with federal regulations regarding human subjects protection, including the need for institutional review and oversight. These requirements ensure that patient safety remains at the forefront of research considerations, reinforcing the importance of ethical practices within funded studies. In this way, NIH funding serves as a regulatory backbone that helps enforce ethical standards across the landscape of medical research.
On the flip side, funding cuts pose significant risks not only to the research itself but also to the ethical frameworks surrounding it. As federal budgets tighten and funding is reduced, researchers may find themselves constrained in their ability to uphold the necessary ethical standards associated with human-subject research. The elimination of essential funding can lead to staff layoffs, reduced IRB operations, and diminished resources for necessary training. As a result, the ability to conduct research that is both innovative and ethically sound could be severely compromised, undermining the essential patient safety measures that are supposed to be in place.
Lastly, to sustain rigorous research ethics, ongoing advocacy for federal research funding is necessary. Policymakers and stakeholders must recognize the vital connection between sufficient funding and the promotion of ethical practices in medical research. By championing NIH funding and ensuring that resources are allocated wisely, we can fortify the integrity of research ethics and enhance protections for patient safety, ensuring a brighter and safer future for all participants in medical studies.
Frequently Asked Questions
What is patient safety in medical research and how is it ensured?
Patient safety in medical research refers to the measures taken to protect the rights and well-being of individuals participating in clinical studies. This is primarily ensured through the oversight of Institutional Review Boards (IRBs), which evaluate research proposals for ethical compliance and participant protection. IRBs assess research design, informed consent processes, and risk mitigation strategies to prevent harm. Regular funding from sources like the NIH supports these oversight functions, ensuring that ethical standards in medical research are upheld.
How does NIH funding impact patient safety in medical research?
NIH funding plays a crucial role in enhancing patient safety in medical research by providing necessary resources for IRB operations and ethical oversight. The NIH mandates that research involving human participants must adhere to strict review protocols to ensure participant welfare. This funding helps facilitate the establishment of systems like the SMART IRB, promoting efficient oversight across multiple research sites and ensuring ethical compliance, which ultimately safeguards the rights of participants.
What role do Institutional Review Boards (IRBs) play in ensuring patient safety in medical research?
Institutional Review Boards (IRBs) are essential for ensuring patient safety in medical research. They conduct thorough reviews of research proposals, focusing on ethical standards such as informed consent, risk assessment, and participant welfare. By monitoring ongoing studies, IRBs help mitigate potential harms and ensure that the rights of research participants are protected throughout the research process. Their oversight is crucial for maintaining public trust in medical research.
What are the consequences of funding cuts on patient safety in medical research?
Funding cuts can significantly jeopardize patient safety in medical research by disrupting the oversight mechanisms provided by IRBs. Without adequate funding, ongoing studies may be halted, new clinical sites may not participate, and ethical monitoring may diminish. This can lead to increased risks for research participants, heightened public skepticism about medical studies, and hindered collaboration between research institutions, ultimately threatening the integrity of patient safety protocols.
How do historical precedents inform the current practices of patient protection in medical research?
Historical events, such as the Tuskegee syphilis study and unethical medical experiments during World War II, have profoundly shaped current patient protection practices in medical research. These instances highlighted the need for robust oversight mechanisms like IRBs to safeguard participant rights and welfare. The establishment of ethical standards and regulations in response to these abuses aims to prevent future violations and ensure that informed consent and safety are prioritized in research involving human subjects.
What is the SMART IRB system and how does it enhance patient safety in medical research?
The SMART IRB system is a national framework designed to streamline compliance and oversight in multi-site research by providing a single Institutional Review Board for collaborative projects. Managed by a team from Harvard Catalyst, this system enhances patient safety by ensuring consistent ethical review across participating institutions. It reduces the administrative burden on researchers, allowing for quicker approvals while maintaining strict standards for protecting research participants.
How can medical research ethics oversight improve patient safety?
Medical research ethics oversight improves patient safety by enforcing regulations that protect individuals participating in clinical studies. Oversight bodies, such as IRBs, meticulously review research proposals, ensure informed consent procedures are followed, assess potential risks, and monitor ongoing trials for compliance. This comprehensive approach fosters an environment that prioritizes participant welfare and ethics in research practices, ultimately contributing to safer and more trustworthy medical advancements.
| Key Point | Explanation |
|---|---|
| Impact of Funding Cuts | Funding cuts disrupt the oversight system critical for safeguarding patient rights and safety in medical research. |
| Role of IRBs | Institutional Review Boards (IRBs) are essential in overseeing research proposals to ensure ethical treatment and protection of participants. |
| Regulatory Developments | The NIH mandates multi-site research to be reviewed by a single IRB to streamline oversight and enhance patient safety. |
| Historical Context | Historical abuses in medical research have prompted the establishment of rigorous ethical standards and IRBs to prevent harm. |
| Consequences of Disruption | Disruption in funding risks delays in research and can lead to increased public skepticism and mistrust in medical research. |
Summary
Patient safety in medical research has become increasingly compromised due to recent funding disruptions. The halt of federal research grants has led to significant challenges in maintaining the oversight that is vital for protecting the rights and welfare of participants in clinical studies. As this situation evolves, the ramifications for both trust in the research process and the health of individuals participating in studies continue to grow, urging a call to action for restoring adequate funding and oversight.