Microglial research has emerged as a pivotal frontier in neuroscience, particularly in understanding the underlying mechanisms of Alzheimer’s disease and other neurodegenerative diseases. These specialized immune cells function as the brain’s protective agents, tirelessly patrolling for signs of damage and playing a critical role in synaptic pruning, the process through which neurons refine their connections. Contributions from pioneering scientists like Beth Stevens have illuminated how malfunctions in microglial activity can lead to devastating ramifications, such as improper pruning directly linked to cognitive decline and memory loss. The ongoing investigations into these fascinating cells not only open new avenues for potential treatments but also provide invaluable insights into the brain’s immune response and overall function. As researchers continue to unveil the intricate relationship between microglia and neurodegenerative conditions, their work holds promise for improving the lives of millions grappling with Alzheimer’s and similar ailments.
Investigating the role of brain microglia has become essential in modern neuroscience, shedding light on an often-overlooked component of the central nervous system’s immune response. This research delves into how these cells maintain neural health by regulating synaptic connections throughout the brain, forming a bridge between immunology and neurobiology. With a focus on synaptic regulation and the implications of microglial dysfunction, scientists aim to uncover potential biomarkers for neurological diseases, particularly concerning Alzheimer’s and various other degenerative disorders. As the landscape of brain immune system studies evolves, researchers like Beth Stevens are leading us toward deeper understanding and innovative therapeutic strategies. The exploration of microglial behavior not only enhances our grasp of ordinary brain function but also provokes critical discussions around treatment possibilities for those affected by debilitating cognitive diseases.
The Role of Microglial Research in Alzheimer’s Disease
Microglial research has gained significant attention in the realm of neurodegenerative diseases, particularly in understanding Alzheimer’s disease. Microglia are specialized immune cells in the brain that play a critical role in maintaining brain health by clearing out dead neurons and facilitating synaptic pruning, a process essential for cognitive function. In recent years, scientists like Beth Stevens have uncovered how dysfunctional microglial activity contributes to the progression of Alzheimer’s disease. This deepening understanding is essential for developing innovative therapeutic strategies to combat the disease.
The focus on microglial cells has revealed that these immune warriors not only assist in removing cellular debris but also influence the strength and formation of synapses between neurons. When microglial function becomes aberrant, as shown in conditions like Alzheimer’s and Huntington’s diseases, it can lead to excessive synaptic pruning and ultimately neurodegeneration. This highlights the importance of microglial research as a promising avenue for identifying early biomarkers and potential treatment modalities that could significantly alter the trajectory of Alzheimer’s, affecting millions of lives.
Impact of Neuroinflammation on Neurodegenerative Diseases
Neuroinflammation is increasingly recognized as a pivotal mechanism in the development of neurodegenerative diseases. Research has demonstrated that chronic inflammation in the brain, often mediated by microglial cells, can exacerbate the progression of Alzheimer’s disease. When neuroinflammation persists, it can lead to cellular dysfunction, impaired synaptic communication, and neuronal damage. Understanding the inflammatory pathways and how microglia regulate these processes is crucial in developing effective interventions for diseases characterized by neurodegeneration.
Beth Stevens and her research team have been at the forefront of exploring the implications of neuroinflammation. By investigating how microglia shift from a protective role to a detrimental one, they are unveiling new strategies for intervention. For instance, targeting specific pathways that modulate microglial activation could potentially dampen neuroinflammation, offering a therapeutic strategy that may slow or halt the progression of Alzheimer’s and related disorders. This area of research not only enhances our understanding of the disease mechanisms but also opens doors for novel therapeutic approaches.
Synaptic Pruning and Its Relevance to Brain Health
Synaptic pruning is a natural process through which unused neural connections are eliminated, allowing the brain to function more efficiently. In healthy brain development, microglia facilitate this pruning, sculpting the neural circuits that underpin cognitive abilities. However, in the context of Alzheimer’s disease, improper synaptic pruning can occur. This dysregulation can lead to the loss of essential synapses, contributing to cognitive decline. The relationship between synaptic pruning and brain health cannot be overstated, as an imbalance in this process often signals underlying neurodegenerative changes.
Through her innovative work, Beth Stevens illustrates the complexity of synaptic pruning and its pivotal role in neurodevelopment and neurodegeneration. By understanding the mechanisms regulating synaptic pruning, researchers can identify the factors that trigger excessive synaptic loss in diseases like Alzheimer’s. This area of study is crucial for developing therapeutic interventions aimed at restoring normal synaptic function and preserving cognitive health in aging populations. As research evolves, the insights gained from synaptic pruning will be integral in informing new treatment strategies for neurodegenerative diseases.
Discoveries in Neurodevelopment through Microglial Studies
The study of microglia has profoundly impacted our understanding of neurodevelopmental processes. Early research by scientists like Beth Stevens has shown that microglia do not just play a role in immune defense but are also key players in shaping the brain’s architecture during critical developmental stages. This discovery has changed the narrative around how we understand brain development and the factors that can influence it, especially in the context of diseases like Alzheimer’s and autism spectrum disorders.
Investigating how microglia interact with developing neurons has unveiled the subtle ways in which the brain establishes and refines neural connections. These insights are crucial because disruptions in the microglial function can lead to developmental disorders and neurodegenerative diseases later in life. As ongoing research continues to explore these dynamics, it is clear that understanding the role of microglia will be essential in informing future therapeutic approaches that target early developmental stages to potentially prevent the onset of neurodegenerative diseases.
The Future of Biomarkers in Alzheimer’s Research
The search for reliable biomarkers in Alzheimer’s disease has become a cornerstone of contemporary research efforts aimed at improving diagnosis and treatment. Biomarkers derived from microglial research hold particular promise. Researchers are examining microglial activation states and the patterns of synaptic pruning as potential indicators of Alzheimer’s progression. By identifying these biomarkers, scientists hope to establish methods for early detection, allowing for intervention before significant cognitive decline occurs.
As scientists like Beth Stevens continue to unravel the complex interactions between microglial function and neuronal health, new biomarkers could be developed that reflect the underlying pathophysiological processes of Alzheimer’s disease. This progress not only enhances our understanding of disease mechanisms but also paves the way for personalized treatment approaches that cater to the individual’s neurobiological profile. The advancement of biomarkers represents a significant leap forward in the fight against Alzheimer’s, potentially altering the course of treatment for millions who struggle with this debilitating condition.
Understanding Neurodegenerative Disease Mechanisms through Microglia
Research into microglial cells has provided a fascinating lens through which to examine the mechanisms of neurodegenerative diseases. Understanding how microglia contribute to diseases like Alzheimer’s unveils the intricate balance between neuroprotection and neurodegeneration. Insights gained from microglial research enable scientists to pinpoint the exact pathways that lead to neuronal loss and cognitive decline, facilitating the design of targeted therapies that could reverse or mitigate the effects of neurodegenerative processes.
Beth Stevens’ work emphasizes the dual nature of microglia as both protectors and potential aggressors in the brain. By exploring the triggers that push microglia toward harmful states, researchers can identify therapeutic targets that could prevent the onset of neurodegenerative diseases. This groundbreaking research demonstrates that by understanding the cellular environment in the brain, we can develop strategies that enhance neuronal survival and function, ultimately improving outcomes for patients suffering from disorders like Alzheimer’s.
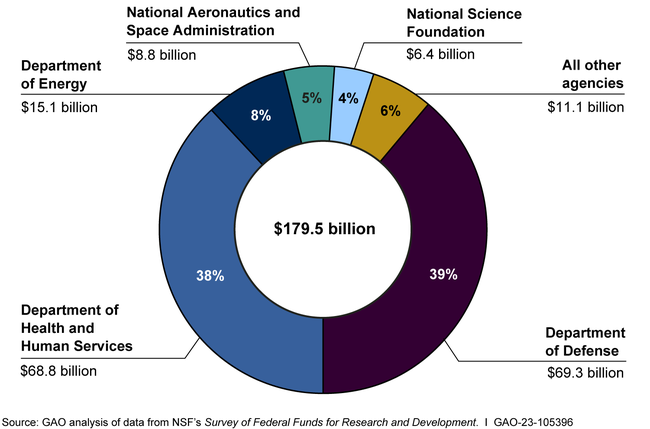
The Importance of Federal Funding in Neurodegenerative Research
Federal funding has been a critical driver of research in neurodegenerative diseases, providing the resources necessary for scientists to explore complex topics such as microglial function in Alzheimer’s disease. Grants from organizations like the National Institutes of Health have enabled researchers like Beth Stevens to pursue innovative studies that significantly enhance our understanding of brain health and disease. This support is essential for fostering curiosity-driven research that leads to groundbreaking discoveries.
The alignment of federal funding with research priorities in neurodegenerative diseases underscores the importance of investing in scientific inquiry that addresses pressing health challenges. Such funding not only supports existing projects but also inspires new generations of scientists to explore the intersections of immunology, neurobiology, and therapeutic development. The ongoing commitment to funding in these areas will be vital for unlocking new avenues of treatment for diseases like Alzheimer’s, promising a brighter outlook for millions of affected individuals.
Beth Stevens: Pioneer in Microglial Research
Beth Stevens stands out as a leading figure in the field of microglial research, making substantial contributions to our understanding of the immune system in the brain and its implications for neurodegenerative diseases. Her pioneering work has illuminated the critical role that microglia play in synaptic pruning and how their dysfunction can lead to conditions such as Alzheimer’s disease. Stevens’ journey from curiosity-driven research to recognized breakthroughs exemplifies the impact that dedicated scientists can have on understanding complex biological systems.
Her recognition as a MacArthur ‘genius’ not only highlights her individual accomplishments but also emphasizes the importance of continued exploration in neuroscience. By fostering a greater understanding of microglial cells, her research serves as a foundation for developing new therapies aimed at mitigating the effects of neurodegenerative diseases. As more research emerges from her lab, it will continue to shed light on intricate neural processes, potentially revolutionizing treatments for Alzheimer’s and similar disorders.
Transformative Potential of Brain Immune System Research
Research into the brain’s immune system, particularly focusing on microglia, holds transformative potential for our approach to neurodegenerative diseases. Understanding the role of these immune cells in maintaining homeostasis and responding to pathology provides a new paradigm for therapeutic strategies. As scientists delve deeper into the interactions between microglia and neurons, they uncover opportunities to harness these interactions for healing and repair, rather than allowing the immune response to contribute to disease progression.
This transformative research not only impacts our grasp of Alzheimer’s disease but also informs broader approaches to brain health management. By investigating how microglia clear debris and modulate synaptic pruning, researchers aim to develop treatments that could enhance neural resilience and protect against cognitive decline. Such innovations could revolutionize the landscape of neurotherapeutics, leading to improved quality of life for individuals suffering from various neurodegenerative conditions.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease and neurodegenerative diseases?
Microglial cells serve as the brain’s immune system, playing a crucial role in detecting and responding to illness or injury. In Alzheimer’s disease, they help clear out damaged cells and coordinate synaptic pruning, a process that, when disrupted, can contribute to neurodegenerative diseases. Research shows that improper microglial activity can worsen the progression of conditions like Alzheimer’s and Huntington’s disease.
How has Beth Stevens contributed to the field of microglial research?
Beth Stevens has significantly advanced our understanding of microglial cells, uncovering their essential functions in synaptic pruning and brain health. Her research has highlighted how these immune cells are crucial in the development of neurodegenerative diseases, including Alzheimer’s. By identifying the links between microglial activity and disease, her work paves the way for potential biomarkers and treatments for individuals affected by these conditions.
What is the significance of synaptic pruning in microglial research?
Synaptic pruning, facilitated by microglial cells, is vital for normal brain development and function. In her research, Beth Stevens has shown that while synaptic pruning is essential for healthy neural circuits, improper pruning can lead to neurodegenerative diseases like Alzheimer’s disease. Understanding this process helps researchers develop strategies to mitigate the effects of these diseases.
What breakthroughs have been made in microglial research regarding Alzheimer’s disease?
Recent breakthroughs in microglial research, especially from the Stevens Lab, have clarified how microglial dysfunction contributes to Alzheimer’s disease. By better understanding how these cells can improperly prune synapses, researchers aim to develop new biomarkers for early detection and potential therapeutic approaches to slow disease progression.
How does microglial research impact the treatment of neurodegenerative diseases?
Microglial research directly impacts the treatment of neurodegenerative diseases by revealing the mechanisms behind immune responses in the brain. Discoveries made by researchers like Beth Stevens have identified potential therapeutic targets, suggesting that modulating microglial activity could lead to improved outcomes for diseases such as Alzheimer’s.
What challenges do researchers face in microglial research related to Alzheimer’s?
Researchers in microglial research face challenges such as the complexity of brain immune responses and the need for innovative methodologies to study microglia in their natural environment. As highlighted in Beth Stevens’ work, understanding the delicate balance of microglial functions is essential, as both overactive and underactive microglial responses can contribute to neurodegenerative processes.
Can changes in microglial activity predict the onset of Alzheimer’s disease?
Emerging evidence suggests that changes in microglial activity may serve as indicators of Alzheimer’s disease onset. The work of researchers like Beth Stevens is focused on identifying specific markers of microglial dysfunction that could help in the early diagnosis and intervention of Alzheimer’s and other neurodegenerative diseases.
How does the funding for microglial research improve Alzheimer’s disease knowledge?
Funding from organizations like the National Institutes of Health plays a critical role in advancing microglial research. It supports groundbreaking studies that enable scientists to investigate the roles of microglia in Alzheimer’s and other neurodegenerative diseases, leading to better understanding and innovative treatment strategies.
| Key Point | Details |
|---|---|
| Microglial Cells | Act as the brain’s immune system, patrolling for signs of illness and injury. |
| Role in Pruning | They clear out dead or damaged cells and prune synapses essential for neuron communication. |
| Misguided Pruning | Improper pruning is linked to Alzheimer’s, Huntington’s disease, and other disorders. |
| Research Impact | Foundational research leads to new biomarkers and potential treatments for neurodegenerative diseases. |
| Funding and Support | Significant federal funding, especially from the NIH, has been crucial for progress in microglial research. |
| Curiosity-Driven Research | Innovative science in basic research can lead to understanding complex diseases. |
Summary
Microglial research is vital for advancing our understanding of neurodegenerative diseases like Alzheimer’s. Beth Stevens, a pioneering researcher in this field, highlights the importance of foundational curiosity-driven studies that explore the immune functions of microglia in the brain. This research not only uncovers how these immune cells contribute to healthy brain function but also how their dysfunction can lead to significant health challenges. By continuing to investigate the role of microglia, scientists hope to unveil new therapeutic avenues that can ultimately enhance the quality of life for millions affected by Alzheimer’s disease and similar conditions.