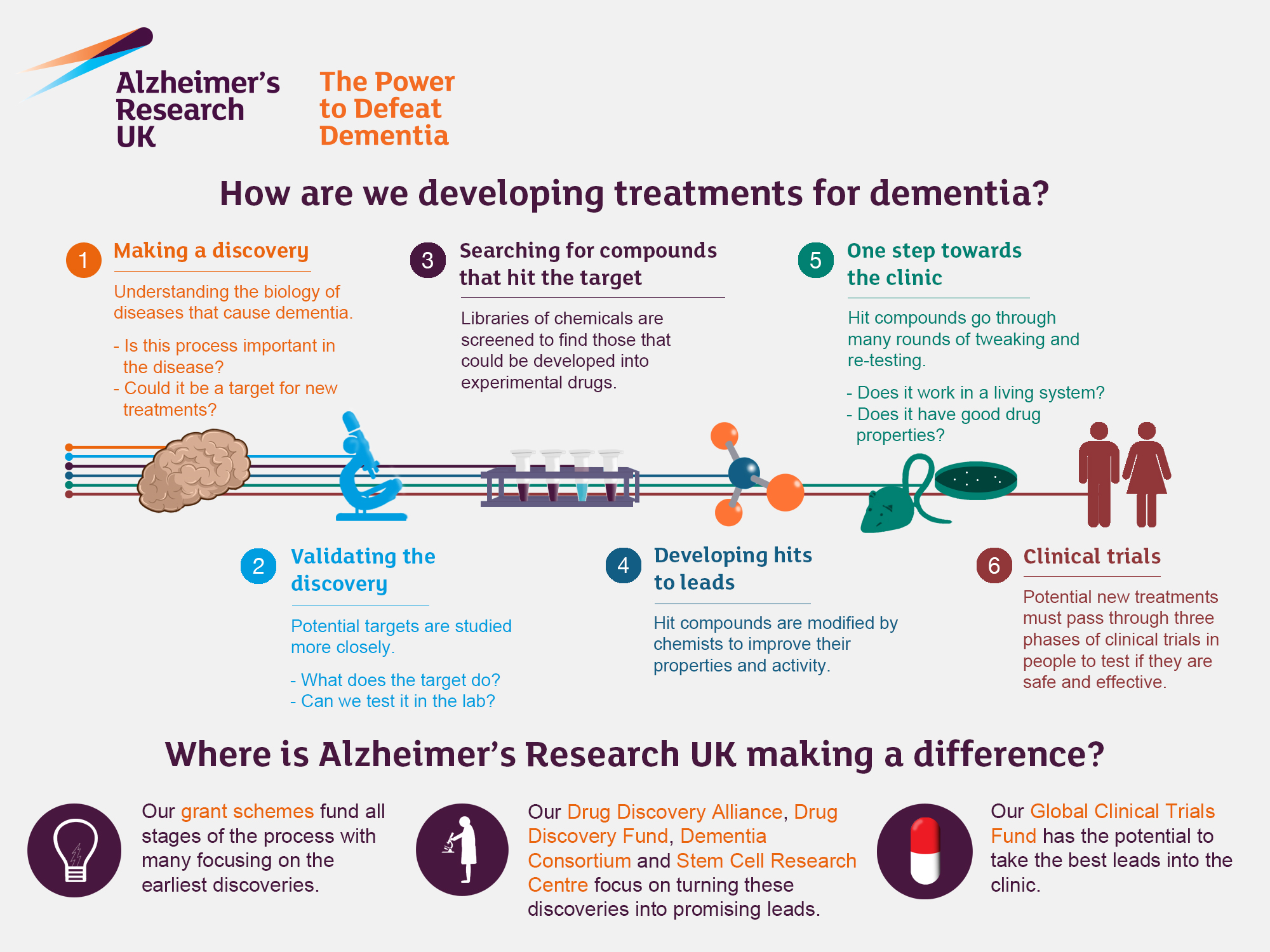
Alzheimer’s research is at the forefront of neuroscientific innovation, as experts like neuroscientist Beth Stevens uncover the intricate roles played by microglial cells in the brain’s immune response. These specialized cells are essential for maintaining neural health by clearing away dead cells and optimizing synaptic connections. However, Stevens and her team have discovered that abnormal pruning by these microglial cells can contribute to devastating conditions such as Alzheimer’s disease and Huntington’s disease. Their groundbreaking findings pave the way for potential treatments for Alzheimer’s and provide crucial insights for addressing other neurodegenerative diseases. As the population ages and the prevalence of Alzheimer’s escalates, the urgency of this research becomes increasingly pronounced, promising to significantly influence the future of healthcare for millions.
The exploration of Alzheimer’s disease encompasses a broad spectrum of complex neurological challenges. Known also as senile dementia, this condition has drawn significant attention from researchers aiming to unveil the underlying mechanisms that drive cognitive decline. Leading the charge in this area is Dr. Beth Stevens, who has revolutionized our understanding of how immune cells, particularly microglia, affect brain health and disease progression. By investigating the role of these cells in neurological disorders, the implications for therapies targeting not only Alzheimer’s but also related neurodegenerative disorders are vast. As we deepen our insights into such critical aspects of brain function, the potential for novel treatments and improved patient outcomes becomes increasingly bright.
Understanding the Role of Microglial Cells in Alzheimer’s Disease
Microglial cells are crucial components of the brain’s immune system, focused on maintaining homeostasis and protecting neural environments. These specialized cells survey the central nervous system, identifying and eliminating pathogens and debris such as dead neurons. In the context of Alzheimer’s disease, however, their function can become disrupted, leading to harmful overactivity. Neuroscientist Beth Stevens has highlighted that when microglial cells malfunction, they may prune synapses excessively, which can contribute to the disease’s progression. Research shows that this abnormal pruning not only affects cognitive functions but also plays a significant role in the pathophysiology of other neurodegenerative diseases, including Huntington’s disease. Understanding these mechanisms is critical for developing targeted treatments for Alzheimer’s and enhancing brain health overall.
Further, Stevens’ work emphasizes the importance of microglial cells in the context of neuroinflammation, a characteristic feature of Alzheimer’s disease. By identifying the triggers that lead to microglial activation, researchers can uncover potential biomarkers for early diagnosis. The identification of these biomarkers could revolutionize Alzheimer’s treatment strategies, allowing for intervention long before overt symptoms appear. This aligns with a growing focus in neuroscience: the need to explore how the immune response within the brain influences neurodegenerative processes. By delving deeper into the intricate relationship between microglial activity and Alzheimer’s progression, researchers hope to pave the way for innovative and effective therapies.
The Future of Alzheimer’s Research and Treatment Advances
Alzheimer’s research stands at a pivotal juncture, bolstered by findings from pioneers like Beth Stevens. As the understanding of microglial cells expands, there is optimism that future treatments may directly target the underlying pathological mechanisms of the disease. The insights gained into abnormal synaptic pruning set the stage for developing novel pharmacological agents that could help regulate microglial function. In turn, such treatments might not only halt disease progression but also preserve cognitive functions in individuals afflicted with Alzheimer’s. This proactive approach aligns with the broader goals of personalized medicine, which aims to tailor interventions based on individual biological markers unique to each patient.
In addition to pharmacological advancements, interdisciplinary collaboration will be vital in the fight against Alzheimer’s disease. The integration of neurobiology, genetics, and even community health perspectives can create a more comprehensive understanding of the disease. For instance, incorporating lifestyles and environmental factors into research on Alzheimer’s could uncover new risk factors and preventive strategies. As the population ages, harnessing such multifaceted approaches becomes essential for addressing the looming Alzheimer’s crisis. Collaborative efforts among organizations and research groups can amplify findings, further accelerating the translation of groundbreaking research into meaningful treatments that improve the lives of millions.
Key Research Insights from Beth Stevens’ Lab
Beth Stevens’ lab at Boston Children’s Hospital is leading crucial research into how microglial cells affect various neurodegenerative diseases, including Alzheimer’s and Huntington’s disease. The lab’s groundbreaking studies have explored how the immune functions of these cells relate to synaptic health. By uncovering the mechanisms of how microglia interact with neurons during both healthy and pathological states, Stevens and her team are providing essential insights that could inform the development of new therapies. This foundational research not only enhances our understanding of Alzheimer’s but also encourages researchers to explore similar immune-related pathways in other neurodegenerative conditions.
The implication of Stevens’ research goes beyond basic science; it sets the groundwork for future innovations in medical technology. By pinpointing specific pathways affected by microglial cells, scientists can create targeted therapies aimed at restoring normal functioning in the brain. For instance, addressing the inflammatory responses initiated by dysfunctional microglia could lead to treatments that not only alleviate Alzheimer’s symptoms but also potentially reverse neurodegeneration. This promising perspective highlights the potential for translating scientific discoveries into practical healthcare solutions in the fight against Alzheimer’s disease.
The Importance of Funding in Alzheimer’s Research
Federal funding has been pivotal in advancing Alzheimer’s research, especially in the early stages of many significant studies. Beth Stevens emphasizes the role of the National Institutes of Health (NIH) and other government agencies in supporting innovative research that might otherwise struggle to gain traction. Financial backing provides researchers not only with the resources they need to conduct experiments but also with the freedom to pursue exploratory inquiries that could lead to groundbreaking discoveries. This support is vital as scientists like Stevens investigate complex processes such as microglial functionality and their implications for Alzheimer’s and other neurodegenerative diseases.
As the aging population continues to grow, the demand for effective Alzheimer’s treatments is becoming increasingly urgent. Rigorous funding in Alzheimer’s research can ensure that future studies maintain momentum, ultimately leading to the development of innovative therapies. Increased investment in basic science and its translation into clinical practice holds the promise of revolutionizing healthcare outcomes for Alzheimer’s patients. As researchers make strides in understanding the disease, robust funding will remain a crucial player in crafting strategies to combat Alzheimer’s and improve the lives of millions battling neurodegenerative disorders.
Advancements in Biomarkers for Early Detection of Alzheimer’s
One of the most exciting prospects in Alzheimer’s research is the development of biomarkers for early detection. This approach is critical as early intervention may dramatically alter the disease trajectory. The work of Beth Stevens and her colleagues illustrates how pinpointing specific immune responses associated with microglial dysfunction could lead to novel biomarkers. By identifying these markers, healthcare providers could screen patients more effectively and begin treatment at the earliest stages of the disease. This shift towards prevention lies at the heart of modern Alzheimer’s research, aiming to reduce the societal impact of the disease significantly.
Moreover, the search for biomarkers is complemented by the potential to track disease progression more accurately. Understanding how microglial activity correlates with cognitive decline can inform treatment adjustments, leading to personalized care strategies. Furthermore, as researchers continue to unravel the complexities of Alzheimer’s, integrating biomarker data with other diagnostic tools will create a more holistic view of a patient’s condition. This confluence of advancements could empower both clinicians and patients, ultimately leading to improved management of Alzheimer’s disease.
Innovations in Neurodegenerative Disease Research
The research landscape for neurodegenerative diseases is rapidly evolving, with significant contributions from neuroscientists like Beth Stevens. Innovations in understanding microglial roles have opened new avenues in exploring various forms of dementia, including Alzheimer’s and Huntington’s disease. By employing cutting-edge techniques such as advanced imaging and genomic profiling, researchers are now able to examine the cellular interactions in the brain more closely. These innovations facilitate deeper insights into how microglial dysfunction can predate visible symptoms of diseases, highlighting the potential for early therapeutic interventions.
Additionally, interdisciplinary approaches are fostering new collaborations that enhance the depth of research efforts. By bridging fields such as biology, pharmacology, and informatics, scientists can design more comprehensive studies that account for the multifactorial nature of neurodegenerative diseases. This holistic outlook is crucial, as it combines knowledge from diverse areas to build integrated models of disease progression. As innovations continue to unfold, the potential to discover effective treatments for Alzheimer’s and other neurodegenerative disorders grows stronger, promising a brighter future for patients and families affected by these conditions.
Understanding the Impact of Aging on Alzheimer’s Disease
Aging is the most significant risk factor for developing Alzheimer’s disease, with increasing age correlating strongly with the incidence of this debilitating condition. As the population ages, understanding the biological underpinnings of Alzheimer’s has become a priority for researchers. Beth Stevens’ work on microglial cells elucidates how aging impacts these immune cells and, consequently, brain health. Changes in microglial function with age can lead to an exacerbated inflammatory state, making the brain more vulnerable to neurodegeneration. This knowledge is crucial for developing age-specific therapeutic strategies that address the unique challenges posed by cognitive decline in the elderly.
Moreover, age-related changes in the brain’s environment can affect not only microglial activity but also overall neuronal health. The interplay between aging and neuroinflammation is a key area of study in Alzheimer’s research, with Stevens advocating for a more nuanced understanding of these relationships. Insights from this research are essential in crafting interventions that target not just the symptoms of Alzheimer’s but also its root causes. By systematically addressing the impacts of aging on brain health, researchers aim to reformulate approaches that could significantly alter the aging trajectory toward Alzheimer’s.
Encouraging Collaboration in Alzheimer’s Research
The complexity of Alzheimer’s disease necessitates diverse expertise, underscoring the importance of collaboration in research efforts. Beth Stevens advocates for interdisciplinary partnerships that bring together specialists from various fields, including neurology, immunology, and genetics. Such collaborations can leverage different perspectives and facilitate comprehensive studies that address multifaceted questions about Alzheimer’s pathology. By pooling resources and insights, researchers can accelerate the pace of discovery and foster innovations that may lead to effective treatments and preventive measures.
Furthermore, initiatives that promote open data sharing and cooperative research frameworks can enhance transparency and build a collective knowledge base. By sharing findings across institutions, researchers can replicate studies, validate results, and ultimately drive progress in understanding Alzheimer’s disease. Public and private sectors, including foundations and governmental organizations, play a crucial role in motivating such collaborations. Through a unified effort, the scientific community can advance Alzheimer’s research more efficiently, expediting the journey from laboratory discoveries to real-world applications.
The Vision for Future Alzheimer’s Treatments
Looking ahead, the vision for Alzheimer’s research is not just about managing symptoms but seeking curative strategies. Research spearheaded by neuroscientists like Beth Stevens emphasizes a paradigm shift towards identifying the underlying biological mechanisms that drive the disease. This includes a focused effort on understanding microglial dysfunction and its contribution to neurodegeneration. As we continue to uncover the complexities of Alzheimer’s, the goal is to create a pipeline for novel treatments that can mitigate disease onset and progression effectively. Ultimately, this vision includes developing vaccines or immunotherapy approaches aimed at restoring normal microglial function.
Simultaneously, embracing technological advancements can further shape the future landscape of Alzheimer’s treatments. Innovations such as machine learning and artificial intelligence hold promise for analyzing data from research studies, streamlining drug discovery processes, and personalizing treatment plans based on individual patient profiles. Integrating these technologies within Alzheimer’s research enables a forward-thinking approach that prioritizes efficacy and patient outcomes. As researchers and clinicians unite their efforts, the hope is to witness a transformative impact on the lives of those affected by Alzheimer’s and pave the way for a future devoid of its burdens.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are crucial to Alzheimer’s research as they function as the brain’s immune system. They help clear away dead or damaged cells and prune synapses, which is vital for maintaining healthy neural connections. Abnormal microglial activity can contribute to neurodegenerative diseases like Alzheimer’s and Huntington’s disease, highlighting their importance in understanding and potentially treating these conditions.
How is Beth Stevens contributing to advancements in Alzheimer’s research?
Neuroscientist Beth Stevens has significantly advanced Alzheimer’s research by exploring how microglial cells prune synapses. Her findings indicate that abnormal pruning may lead to neurodegenerative diseases, including Alzheimer’s. Stevens’ work lays the groundwork for new therapeutic strategies and early detection biomarkers, which are essential for improving treatment outcomes for Alzheimer’s patients.
What implications does Stevens’ research have for treatment for Alzheimer’s?
Stevens’ research on microglial cells provides insights that could lead to novel treatments for Alzheimer’s disease. By understanding how abnormal synaptic pruning contributes to the disease, scientists can develop targeted therapies that mitigate these effects, potentially improving the quality of life for millions of Alzheimer’s patients in the U.S.
Why is it important to study neurodegenerative diseases like Alzheimer’s and Huntington’s disease together?
Studying neurodegenerative diseases like Alzheimer’s and Huntington’s disease together is important because they share common pathways, particularly related to microglial function and synaptic pruning. Insights gained from researching these diseases can lead to a better understanding of their mechanisms, facilitating the development of treatments that address a range of neurodegenerative conditions.
How can understanding microglial cells improve early detection of Alzheimer’s?
Understanding microglial cells can improve early detection of Alzheimer’s by identifying biomarkers related to their abnormal activity. Research by scientists like Beth Stevens indicates that changes in microglial function correlate with disease progression, opening avenues for developing tests that detect Alzheimer’s earlier, which is critical for effective intervention.
What is the future outlook for Alzheimer’s research based on current studies?
The future outlook for Alzheimer’s research is promising, especially with ongoing studies focusing on microglial cells and their role in disease pathology. With advancements in understanding how these immune cells affect neural health, researchers are optimistic about discovering new treatments and interventions that can substantially alter the course of Alzheimer’s and improve patient outcomes.
How does federal funding influence Alzheimer’s research like that of Beth Stevens?
Federal funding is vital for Alzheimer’s research, as highlighted by Beth Stevens’ work, which has relied heavily on support from the National Institutes of Health. Such funding enables scientists to explore fundamental questions and develop innovative approaches to tackle complex neurodegenerative diseases, ultimately leading to breakthroughs in treatments and better preventive strategies.
| Key Points |
|---|
| Neuroscientist Beth Stevens is leading significant research in Alzheimer’s disease. |
| Microglial cells act as the immune system of the brain, crucial for maintaining brain health. |
| Abnormal pruning by microglia is linked to Alzheimer’s and other neurodegenerative diseases. |
| Stevens’ research is foundational for developing new medicines and early detection biomarkers. |
| Federal funding, particularly from the NIH, has been pivotal in supporting her research. |
| The prevalence of Alzheimer’s is expected to double by 2050, stressing the need for advancements in treatment. |
Summary
Alzheimer’s research is being significantly advanced through the pioneering work of neuroscientist Beth Stevens, who is redefining our understanding of microglial cells and their roles in brain health. With improper functioning linked to Alzheimer’s disease, Stevens’ innovative studies are crucial in developing new therapies and early detection methods, which could greatly impact millions affected by this devastating illness.