TIM-3 therapy represents a groundbreaking approach in the fight against Alzheimer’s disease, leveraging insights from cancer treatment strategies to harness the immune system. By targeting the TIM-3 molecule, researchers have discovered a method to unlock the potential of microglia, the brain’s immune cells, which are crucial for clearing plaque associated with Alzheimer’s. This therapy has shown promise in reversing cognitive decline in animal models, highlighting its ability to restore memory and cognitive functions. As the understanding of Alzheimer’s disease deepens, TIM-3 therapy stands out as a beacon of hope, potentially transforming the landscape of Alzheimer’s treatment. With its roots in immune regulation, this innovative therapy could redefine how we approach neurodegenerative diseases in the future.
Referred to as TIM-3 inhibition or immune checkpoint modulation, TIM-3 therapy harnesses the body’s defenses to combat Alzheimer’s disease effectively. By disrupting the function of TIM-3, a key regulatory molecule in the immune response, this novel treatment aims to boost the activity of microglial cells responsible for clearing harmful amyloid plaques in the brain. The implications of this approach are significant, particularly as the immune system’s role in neurodegeneration gains more attention. In contrast to traditional methods that have shown limited success, TIM-3 modulation may offer a more targeted and effective solution for preserving cognitive function and battling Alzheimer’s disease. As research advances, the potential applications of TIM-3 therapy could extend beyond neurodegenerative conditions, potentially influencing cancer treatment paradigms as well.
The Role of TIM-3 Therapy in Alzheimer’s Disease Treatment
Recent studies suggest that inhibiting the checkpoint molecule TIM-3 opens new avenues for treating Alzheimer’s disease, a neurodegenerative condition affecting millions. By disabling TIM-3, researchers found that microglia — the brain’s resident immune cells — can more effectively clear amyloid plaques that contribute to cognitive decline. Improved memory in mouse models signifies the potential of TIM-3 therapy in rejuvenating cognitive function, indicating that targeted treatments could be pivotal in combating late-onset Alzheimer’s.
With Alzheimer’s characterized by a gradual loss of cognitive function, TIM-3 therapy represents a breakthrough strategy previously leveraged in cancer treatments. As scientists delve deeper into the mechanisms of immune response within the brain, the promise of repurposing anti-TIM-3 antibodies for Alzheimer’s treatment is becoming increasingly viable. Despite previous setbacks in drug development for Alzheimer’s, TIM-3’s role in enhancing microglial activity offers hope for more effective interventions against this debilitating disease.
Microglia: Guardians of Brain Health
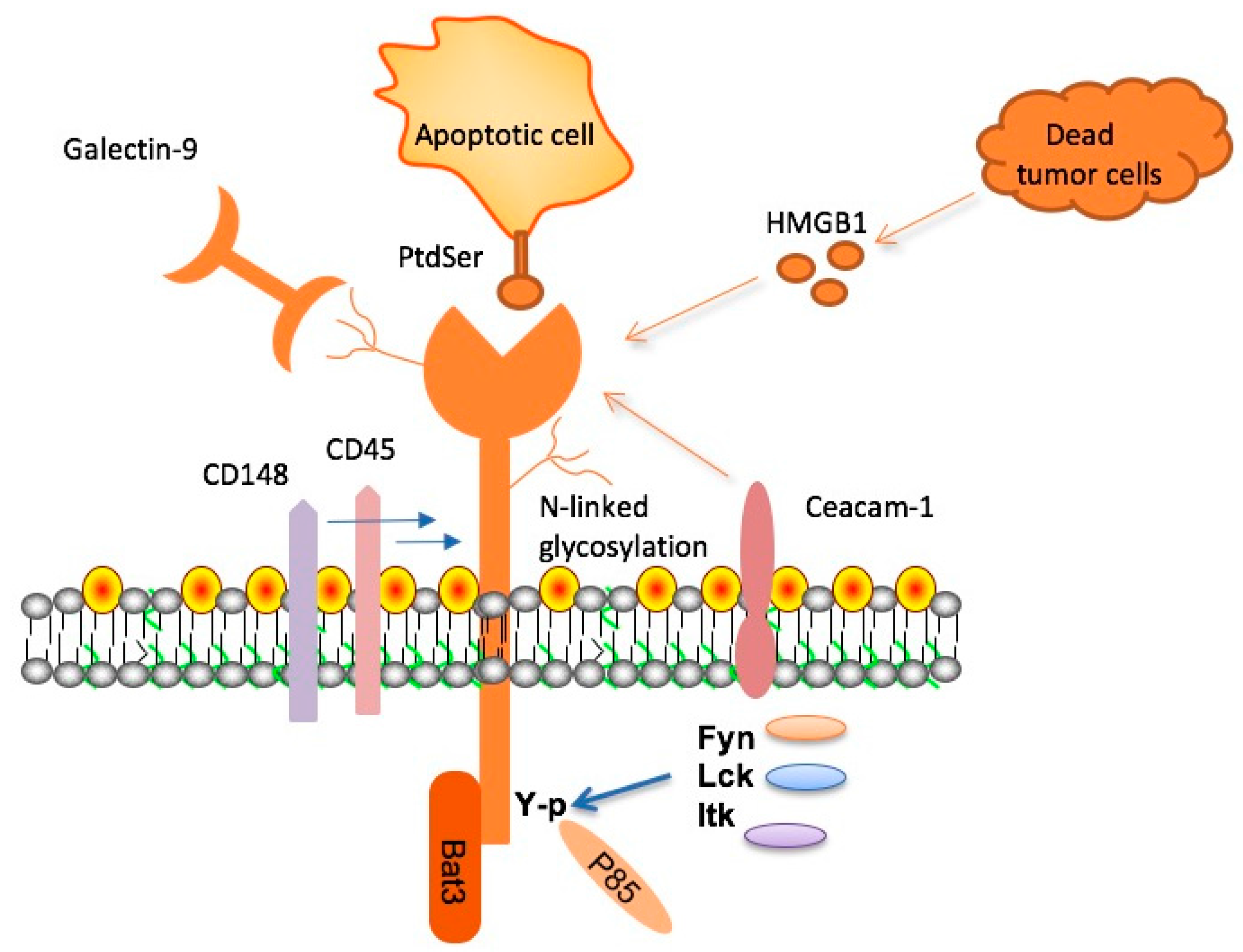
Microglia play a crucial role in maintaining brain health, acting as the immune defense within the central nervous system. These cells are not only responsible for clearing cellular debris and plaques but also for pruning synapses during development, which is essential for cognitive function. However, with age, their efficacy can diminish, particularly in response to plaque formation seen in Alzheimer’s disease, where increased TIM-3 expression can hinder their ability to perform these necessary functions.
The balance in microglial activity is vital; while their initial activation is beneficial to clearing amyloid beta plaques, prolonged activation can lead to a state of homeostasis where they become less responsive. This dysfunctional state is worsened by the accumulation of inhibitory molecules like TIM-3 within Alzheimer’s pathology, leading to plaque build-up and subsequent cognitive decline. Therefore, understanding and manipulating microglial responses are crucial in developing effective Alzheimer’s treatments.
Understanding Checkpoint Molecules in Alzheimer’s Therapy
Checkpoint molecules, particularly TIM-3, are a double-edged sword in immune regulation. While they serve to prevent the immune system from overreacting, which is essential in scenarios like fighting infections, their role in Alzheimer’s disease is more complex. Elevated TIM-3 levels in microglia prevent these cells from attacking harmful amyloid plaques, ultimately exacerbating the disease. Therefore, targeting checkpoint molecules like TIM-3 provides a promising pathway to improve cognitive function by restoring the immune system’s capacity to clear plaques.
In the context of Alzheimer’s, checkpoint inhibitors previously developed for cancer treatment could be adapted to liberate microglia from their inhibitory restraints. As research continues to unravel the connections between immune response and neurodegeneration, it becomes clearer that manipulating these checkpoints could yield therapies that not only halt but potentially reverse the cognitive decline associated with Alzheimer’s.
Implications of Genetic Variants on Alzheimer’s Disease
Genetic factors play a significant role in late-onset Alzheimer’s disease, with specific polymorphisms in genes like HAVCR2, which encodes TIM-3, linked to increased risk. These genetic insights help demystify how certain individuals might have a predisposition to develop Alzheimer’s while others do not. Understanding these genetic underpinnings is crucial for tailoring individualized treatment plans that target the specific biological pathways implicated in Alzheimer’s.
The identification of TIM-3 as a genetic risk factor signifies the importance of genetics in understanding the mechanisms underlying Alzheimer’s disease. This knowledge paves the way for genetic screening as part of early diagnosis and personalized treatment strategies aimed at mitigating the effects of various genetic profiles. As the field progresses, harnessing genetic data will be vital in developing more effective interventions for individuals at risk of developing cognitive impairments.
Exploring Cognitive Function Recovery in Alzheimer’s Models
Recent studies employing mouse models have shown that cognitive functions can significantly improve when TIM-3 is inhibited, allowing microglia to properly clear amyloid plaques. These experiments provide a promising glimpse into potential recovery strategies in Alzheimer’s disease, as mice exhibiting reduced plaque burden regained aspects of memory, which suggests that similar outcomes might be achievable in humans with appropriate interventions.
The ability to measure cognitive behavior through tasks like maze navigation provides critical insights into how treatments can enhance learning and memory. As researchers explore the impacts of TIM-3 therapy, understanding cognitive improvements in preclinical models will inform the design and expectations of human trials, aiming to assess the efficacy and safety of TIM-3 targeted therapies for Alzheimer’s.
Innovative Strategies for Alzheimer’s Drug Development
Revamping drug development strategies is crucial given the disappointments faced by previous trials targeting amyloid plaques directly. The promising results seen with TIM-3 therapies highlight the need for innovative approaches that leverage the immune system’s capabilities. By focusing on biological pathways shared between cancer and Alzheimer’s treatments, researchers are paving the way for more dynamic and effective therapeutic options that address the dual challenges of neurodegeneration and immune dysfunction.
In integrating knowledge from cancer immunotherapy, drug development for Alzheimer’s could benefit significantly from the experiences of checkpoint inhibitors. Existing research on TIM-3 provides rich material for developing new treatment models that may overcome the limitations seen in prior Alzheimer’s drug trials. This innovative strategy signifies a paradigm shift in how we approach neurodegenerative diseases, viewing them through the lens of immune modulation to foster recovery and enhance cognitive function.
Future Directions for Alzheimer’s Research
As scientists continue to uncover the intricate connections between immunity and neurodegeneration, future research must focus on multimodal approaches that integrate genetic, biological, and environmental factors to combat Alzheimer’s disease. The proactive exploration of TIM-3 inhibitors as a therapeutic avenue opens doors to trials aiming to validate the efficacy of immune modulation in human populations.
Moreover, strides in research involving anti-TIM-3 antibodies could significantly alter the therapeutic landscape for Alzheimer’s disease. Ongoing collaborations and studies that explore various combinations of immune therapies could lead to groundbreaking discoveries, ensuring that strategies developed to combat Alzheimer’s are multifaceted, addressing both the underlying causes and symptoms of this harrowing condition.
The Need for Collaborative Research in Neurodegenerative Diseases
Collaboration among research institutions plays a vital role in advancing the understanding of Alzheimer’s disease and developing effective treatments. Brigham and Women’s Hospital and Harvard Medical School exemplify how interdisciplinary teamwork can accelerate findings, like those surrounding TIM-3 and microglial functions. By pooling resources and expertise, researchers can tackle the complexities of neurodegenerative diseases more effectively.
Collaborative efforts extend beyond academia, involving partnerships with pharmaceutical companies, funding agencies, and patient advocacy groups to drive innovation and facilitate the translation of research into clinical practice. This collective action is essential for fostering breakthroughs that improve cognitive function in Alzheimer’s patients, demonstrating how united endeavors can create a more efficient pipeline from laboratory to bedside.
Patient-Focused Approaches in Alzheimer’s Clinical Trials
Designing clinical trials that prioritize patient outcomes is integral as we advance into new Alzheimer’s therapies. With TIM-3 as a prospective target, trials must carefully consider not only the biological effectiveness of treatments but also how they enhance patients’ quality of life. Aligning clinical endpoints with watchfulness for improvements in cognitive function, daily living skills, and overall well-being will form the foundation for future Alzheimer’s research.
Through patient engagement in the trial design, researchers can ensure that the most pressing concerns of those affected are addressed, resulting in more applicable and beneficial treatments. This shift towards patient-centered research encourages active participation, fostering a sense of community and collaboration that is vital for maximizing the impact of emerging therapies in Alzheimer’s care.
Frequently Asked Questions
What is TIM-3 therapy and how does it relate to Alzheimer’s disease?
TIM-3 therapy involves targeting the TIM-3 molecule, which is an immune checkpoint molecule that inhibits the activity of microglia, the brain’s immune cells. Researchers found that by deleting TIM-3 in laboratory mice, microglia were freed to attack amyloid plaques associated with Alzheimer’s disease, leading to improved cognitive function. This suggests that TIM-3 therapy could potentially provide a new approach in treating Alzheimer’s disease by enhancing the brain’s ability to clear toxic plaques.
How does TIM-3 affect cognitive function in Alzheimer’s research?
In studies, TIM-3’s inhibition of microglia has been linked to impaired cognitive function in Alzheimer’s models. The presence of TIM-3 keeps microglia in a homeostatic state, preventing them from clearing amyloid beta plaques that accumulate in Alzheimer’s disease. By using TIM-3 therapy to block this molecule, researchers have observed improved memory and cognitive abilities in mice, indicating a potential therapeutic pathway for enhancing cognitive function in Alzheimer’s patients.
How does TIM-3 therapy compare to traditional Alzheimer’s treatments?
Unlike traditional Alzheimer’s treatments that primarily focus on amyloid beta clearance, TIM-3 therapy represents a novel strategy by enhancing the immune response within the brain. Traditional treatments often face significant challenges due to the blood-brain barrier limiting their effectiveness. In contrast, TIM-3 therapy specifically targets the mechanisms that inhibit microglial activity, which could lead to more effective plaque clearance and cognitive improvements, making it a promising alternative.
What are microglia and what role do they play in TIM-3 therapy for Alzheimer’s?
Microglia are the brain’s resident immune cells responsible for clearing debris, including amyloid plaques associated with Alzheimer’s disease. In the context of TIM-3 therapy, microglia are inhibited by the TIM-3 molecule, preventing them from effectively removing these plaques. By targeting TIM-3, researchers aim to reactivate microglial functions, thereby enhancing their ability to clear amyloid plaques and potentially improving outcomes for Alzheimer’s patients.
What potential does TIM-3 therapy have in cancer treatment as well as Alzheimer’s disease?
TIM-3 therapy has dual potential in both cancer treatment and Alzheimer’s disease due to its role as an immune checkpoint molecule. In cancer, TIM-3 is targeted to enhance T-cell activity against tumors. In Alzheimer’s, TIM-3 is inhibited to promote microglial clearance of plaques. This dual functionality highlights TIM-3 as a versatile target in immunotherapy, with opportunities for developing therapies that address both Alzheimer’s disease and certain cancers.
What future research is needed to advance TIM-3 therapy for Alzheimer’s disease?
Future research on TIM-3 therapy for Alzheimer’s disease will focus on testing human anti-TIM-3 antibodies in relevant mouse models that express the human TIM-3 gene. Further studies will be necessary to evaluate the efficacy and safety of these therapies in clearing amyloid plaques and improving cognitive function. Additionally, understanding the long-term effects and optimizing administration methods for TIM-3 therapy will be critical for its successful application in clinical settings.
What is the significance of the TIM-3 gene polymorphism in Alzheimer’s disease?
The TIM-3 gene polymorphism is significant as it has been associated with an increased risk of developing Alzheimer’s disease. In individuals with this polymorphism, TIM-3 is expressed at higher levels on microglia. This elevated expression inhibits microglial activity and prevents effective plaque clearance, contributing to Alzheimer’s disease progression. Understanding this genetic link is crucial for developing targeted TIM-3 therapies to improve treatment outcomes for at-risk populations.
How long has research on TIM-3 therapy for Alzheimer’s disease been ongoing?
Research on TIM-3 therapy for Alzheimer’s disease has been ongoing for five years, involving extensive experiments to explore its effects. The work is collaborative, employing various genetic models to understand TIM-3’s role better and develop potential therapies for Alzheimer’s. This long-term commitment underscores the complexity of Alzheimer’s research and the dedication required to innovate new treatment avenues.
| Key Points | Details |
|---|---|
| Overview of TIM-3 Therapy | TIM-3 therapy involves modulating the immune response to potentially treat Alzheimer’s disease, similar to its use in cancer treatment. |
| Study Findings | Research indicates that deleting the TIM-3 molecule in microglia can enhance memory and reduce plaque accumulation in mice models of Alzheimer’s. |
| Role of TIM-3 in Immune System | TIM-3 functions as a checkpoint molecule that inhibits microglial activity, preventing them from clearing amyloid plaques, thus worsening Alzheimer’s progression. |
| Implications for Alzheimer’s Treatment | Potential development of anti-TIM-3 antibodies as a therapeutic strategy to restore microglial function and improve cognitive outcomes for Alzheimer’s patients. |
| Research Collaboration | This study was conducted over five years by a collaborative team at Harvard, with promising initial results leading to the next phase of trials. |
Summary
TIM-3 therapy presents a promising avenue for treating Alzheimer’s disease by targeting checkpoint molecules to restore immune cell function. Research has demonstrated that inhibiting TIM-3 can enable microglia to clear amyloid plaques more effectively, potentially reversing cognitive decline associated with Alzheimer’s. This groundbreaking approach may pave the way for new treatments, as ongoing studies explore the effectiveness of anti-TIM-3 antibodies in clinical settings.